Thanks to a new FDA update to tight restrictions that have been in place for over a decade, you no longer have to go to a clinic, medical office, or hospital to pick up abortion pills prescribed by a doctor certified to do so.
You can now pick up or submit a mail order for the medication — mifepristone — at a retail pharmacy, regardless of whether you consult with your healthcare provider virtually or in person.
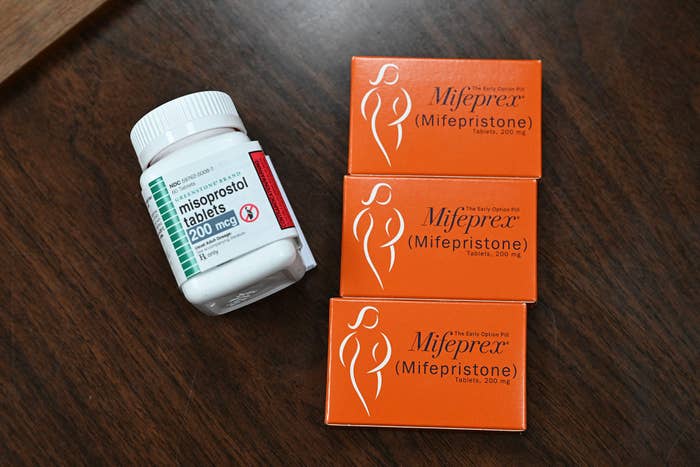
Mifepristone is an oral drug used to end a pregnancy up to 10 weeks gestation, or 70 days or fewer since the first day of a last menstrual period, and is approved to be taken with another medication called misoprostol. The two-drug regimen works by blocking the hormone progesterone, which is needed for a pregnancy to continue, and it is successful at ending early pregnancies 95% of the time. The medication accounts for more than half of all abortions in the US.
The move comes as states continue to limit access to abortion pills and how people can get them following the disastrous overruling of Roe v. Wade last year that stripped people of their right to an abortion in many states.
The FDA’s modification to its risk evaluation and mitigation strategy (REMS) program, officially approved on Tuesday, also added a requirement that pharmacies become certified to dispense the abortion pills by completing a “pharmacy agreement form.”
While the changes are effective immediately, it may take some time before people can reap the benefits as pharmacies decide if they will agree to dispense the medication.
In a statement sent to BuzzFeed News, the pharmacy chain CVS said: “We’re reviewing the FDA’s updated REMS drug safety program for mifepristone to determine the requirements to dispense in states that do not restrict the dispensing of medications prescribed for elective termination of pregnancy.”
Walgreens told us that it looks forward to reviewing the FDA’s update and “will continue to enable [its] pharmacists to dispense medications consistent with federal and state law.”
The Justice Department, in a legal opinion signed Dec. 23 but released late Tuesday, according to reports, said that the US Postal Service can legally mail prescribed mifepristone to people who live in states that have banned access to abortion. The reasoning is that the sender of the drugs cannot know whether the recipient will use them illegally.
“Because there are manifold ways in which recipients in every state may lawfully use such drugs, including to produce an abortion, the mere mailing of such drugs to a particular jurisdiction is an insufficient basis for concluding that the sender intends them to be used unlawfully,” the Justice Department wrote in the opinion.
The FDA said its decision to make these changes was not in response to the Supreme Court’s decision in June that overturned Roe v. Wade and ruled the right to abortion unconstitutional.
Instead, the action is related to Chelius v. Becerra, a federal lawsuit filed in 2017 led by a doctor in Hawaii and several healthcare associations that challenged the constitutionality of the FDA’s REMS program for mifepristone. It argued that the restrictions had no medical basis and placed significant burdens on people with low incomes, people of color, and those living in rural areas because not everyone has equal access to transportation, according to the American Civil Liberties Union.
The requirement for patients to pick up abortion pills in person, despite having been evaluated and counseled virtually via telemedicine, forced people to miss work, have difficulty arranging childcare, and delay care enough to make them ineligible for the medication, the ACLU said. (The FDA had temporarily suspended the in-person requirement during the COVID Public Health Emergency.)
Medical associations like the American College of Obstetricians and Gynecologists, the American Academy of Family Physicians, and the American Medical Association supported the removal of the REMS restrictions.
The lawsuit spurred the FDA to review available data and information, and in December 2021, the agency announced it would remove the in-person dispensing requirement and mandate pharmacies that would offer the pills become certified to do so. The changes, the FDA said at the time, would “reduce the burden on the health care delivery system and ensure the benefits of the product outweigh the risks.”
The new update is a step in the right direction by increasing access to abortion care, but the ACLU notes that the pharmacy certification requirement and the existing requirements that medical professionals be certified to prescribe mifepristone are unnecessary.
(Healthcare providers must be able to accurately date pregnancies and diagnose ectopic pregnancies, which is when a fetus develops outside the womb, such as in the fallopian tubes. All ectopic pregnancies are nonviable and can be deadly for the parent. Providers also must be able to provide necessary surgical intervention or make arrangements for others to provide the care. Patients still have to sign a consent form before receiving their prescription as well.)
“We are happy the FDA has increased pharmacy access to this safe and effective medication, relieving one of the agency’s needless burdens on mifepristone patients. But leading medical groups have long called for outright elimination of the FDA’s special restrictions on mifepristone, which obstruct abortion and miscarriage patients from accessing the time-sensitive health care they need without a corresponding safety benefit,” the ACLU said in response to the FDA update. “As abortion access evaporates across the country, and communities of color and people with fewer resources disproportionately suffer the severe medical consequences of forced pregnancy, the FDA’s actions — while inching toward progress — fall short of what science and justice demand.”
