The FDA has issued three warning letters to U.S. firms claiming that their products can help prevent, treat and cure the deadly Ebola virus plaguing West Africa.
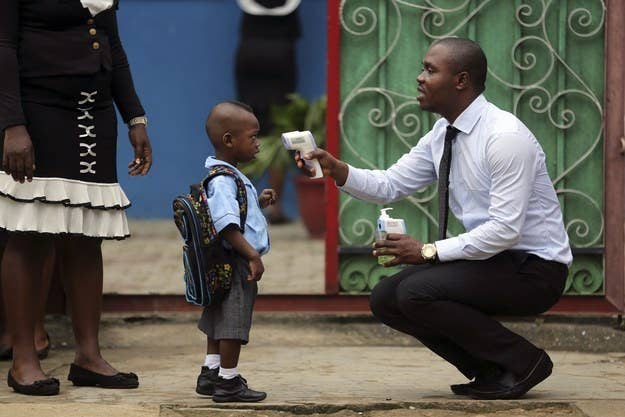
"There are currently no FDA-approved vaccines or prescription or over-the-counter drugs to prevent or treat Ebola," the agency said on Wednesday.
"Individuals and companies promoting these unapproved and fraudulent products must take immediate action to correct or remove these claims or face potential FDA action," the FDA said on its website.
Updated — Sept. 25, 2014 1:17 p.m.
The FDA told BuzzFeed News that they conducted internet surveillance and monitored consumer complaints to identify the companies making fraudulent claims about the cure and prevention of Ebola.
"Since the outbreak of the Ebola virus in West Africa, the FDA has seen and received consumer complaints about a variety of products claiming to either prevent the Ebola virus or treat the infection," Stephanie Yao, FDA's Acting Deputy Director of Strategy, said in an email to BuzzFeed News.
Responding to questions about Nigeria's rejection of one such phony product after the health minister proposed it as an experimental Ebola drug, Yao said the FDA "initiated contact with some foreign governments to convey the agency's concern about these products." However, the FDA did not discuss this topic with these governments until after they had rejected the product, Yao added.
The FDA will continue to monitor consumer complaints and the identified companies to ensure they don't market their fake products under a different name or website. Firms that do not comply with the FDA's requirements could be seized and/or face criminal prosecution, Yao said.
These are the three companies that have claimed their products can prevent, treat or cure Ebola, the deadly virus that has killed nearly 3,000 people in West Africa so far.



1. dōTERRA is a Utah-based company offering therapeutic essential oils.

Their products are sold exclusively by "independent product consultants" who work from home to educate and sell their oils.
In a YouTube video, Mr. Skinny Medic says dōTERRA's "anti-viral" oils could prevent people from contracting the Ebola virus in case of a breakout.

The FDA letter states that dōTERRA oils are also marketed through the Anytime Essentials website run by a "wellness advocate" called Candace Anderson who describes herself as a "Wife to Steve; Mom to five boys."


One of Anytime Wellness's blog posts called "Fight Your Virus with Essential Oils" lists the oregano essential oil as effective in fighting the Ebola virus among other medical conditions.

UPDATE — Sept. 25, 2014 11:50 a.m.: In an email to BuzzFeed News, Candace Anderson said that dōTERRA "absolutely did not pay me to promote their oils as a preventive measure for Ebola."
She said she wrote her blog post prior to Ebola being in the news and cited the fifth edition of the book Modern Essentials in stating the primary use of oregano oil is for the Ebola virus. "It is important for people to know that essential oils have scientifically proven antiviral properties, but unfortunately my site has now been taken down," Anderson said.
As the FDA letter notes, dōTERRA oils are marketed as products to treat various medical conditions — including Alzheimer's, autism, and cancer — by individuals who are not medical practitioners.
The letter notes that marketing of these essential oil products as drugs without FDA approval is in violation of the Federal Food, Drug, and Cosmetic Act.
2. New Jersey-based Natural Solutions Foundation describes itself as "an exempt international NGO" created to "defend and promote" healthcare and health freedom.

As of Thursday, the firm's website, drrimatruthreports.com, no longer exists. However, the firm's products are being sold on Natural Solutions Marketplace.
Laibow, the medical director of the NGO has claimed that a nutrient called "Nano Silver 10" is the only definitive prevention and solution to cure the Ebola virus.


In this video titled, "No one needs to die from Ebola," Laibow accuses the FDA, WHO and other international organizations of keeping the public in the dark about, what she calls, the only antiviral agent that could cure Ebola.
View this video on YouTube
In the video Laibow says, "Ebola virus is said by the FDA, WHO and other international authorities to have no authorized treatment, no cure, so people who get Ebola are just supposed to lie down and die, painfully. The fact is [they} know perfectly well what I'm about to tell you and they're not telling you...the FDA doesn't have the regulatory authority to approve the nutrient, and the cure and prevention for Ebola is a nutrient."
Users are directed to enter their email addresses to receive Laibow's "groundbreaking protocol against Ebola."

The foundation also uses social media to urge users to order "Nano Silver" before the site is banned.
#PayPal #censorship #healthfreedom #nanosilver #DrRima YOU HAVE TIL MIDNITE TONITE TO ORDER NANO SILVER http://t.co/LdTMMTeWte Pls forward!
Laibow has sent open letters to African and world leaders and international health authorities claiming that the U.S. government has suppressed information on the prevention and cure for Ebola since 2009.

A citizen group in Liberia introduced the "Nano Silver" nutrient promoted by Laibow as having the ability to "deactivate and neutralize the Ebola virus," Front Page Africa reported.

Nigeria's health minister, Onyebuchi Chukwu, had also proposed that a new experimental drug called "Nano Silver" would be used to treat Ebola patients.
However, the Nigerian government rejected "Nano Silver" which the U.S. Environmental Protection Agency considers a pesticide, causing social media backlash by Nigerians.

The FDA's letter to Laibow says her company could face legal action and a cease-and-desist order for violating the Federal Trade Commission Act. It may also require the company to refund consumers.
3. Young Living is a Utah-based company that produces and sells essential oils through its "Young Living Distributors."

One of Young Living's paid oil consultants is a nurse practitioner and a mom who wrote about how the "Thieves" essential oil can help fight Ebola on her website "The Oil Dropper."

Other Young Living consultants' posts on preventing Ebola by applying essential oils appear to be deleted or censored since the FDA letter.

The FDA has given all three companies 15 days to correct their noted violations or face regulatory action.
Correction: An earlier version of this post misstated that the FDA classifies nano-silver as a pesticide. The U.S. Environmental Protection Agency considers nano-silver a pesticide. (9/25/2014 3:33 p.m.)

