SAN FRANCISCO — Internal Juul documents, obtained by BuzzFeed News, call into question claims made by a former Juul executive who sued the e-cigarette giant in October for allegedly covering up a shipment of 1 million “contaminated” nicotine pods.
The explosive lawsuit has drawn widespread attention in the midst of a nationwide lung-injury outbreak linked to vaping products containing tainted THC, and as e-cigarette companies like Juul are increasingly scrutinized for their aggressive marketing toward teens.
According to the confidential report, a batch of the e-liquids showed a visual defect where the chemicals did not mix together properly, causing the appearance of “separation.” A series of tests, run by an external lab hired by Juul, determined that this issue was not a “hazard” and posed “no increased health risks to customers.”
The report states that there were “no serious injuries reported” related to the shipment in question, as well as no “violation of any applicable regulatory or statutory law.” Based on the results, the company concluded it would not need to issue a recall.
The 11-page report, signed by multiple top Juul executives overseeing quality, regulatory, safety, and medical matters, includes a table of test results, dated in March, and a summary page dated in August. According to a source familiar with the document, those executives did not officially approve the report until months after the tests were done, on Oct. 22 — just one week before the lawsuit was filed in US District Court for the Northern District of California.
A Juul spokesperson declined to comment on the document or the date it was signed, but issued a statement that confirmed its findings.
Do you work, or have you worked, at Juul? Contact this reporter at stephanie.lee@buzzfeed.com or stephaniemlee@protonmail.com. Visit tips.buzzfeed.com for other secure ways to get in touch.
The report disputes the highly publicized legal claims made by Siddharth Breja, a former senior vice president of global finance at Juul. As first reported by BuzzFeed News, Breja alleged that he was fired this spring after he internally raised concerns about “contamination” — whose nature he did not specify — in 1 million shipped Juul mint pods.
His case has since grown shakier. His attorney, Harmeet Dhillon, filed a motion this month to stop representing him, and he has not yet found a new lawyer, according to court filings. The case is currently headed toward arbitration.
At this point, it is not clear what evidence, if any, Breja has for his contamination claim. He did not return several interview requests, and a representative for his previous law firm declined to comment. Juul also declined to comment on the possibility of a settlement.
From the beginning, Juul has denied that the pods were contaminated and dismissed the lawsuit as “baseless.” “We take product safety seriously and have implemented rigorous quality controls,” spokesperson Austin Finan said by email.
Breja’s “false” claim came from an email chain that incorrectly referred to the issue as involving “contamination,” Finan wrote. “In fact, this situation was not about contamination, it was about separation, which is what our internal and external tests were designed to analyze.”
The tests in the report focused on properties including nicotine levels, density, acidity, and a visual measure called refractive index. On their own, “to be dramatic, if someone had contaminated the products with a potent contaminant, these tests would never reveal that information,” Michelle Peace, an associate professor of forensic science at Virginia Commonwealth University who has studied e-liquids, told BuzzFeed News by email.
Asked about the possibility of other contamination, Finan said that the batch also passed a battery of routine chemical safety tests not covered in the report. He declined, however, to specify which chemicals they tested for or to provide documentation supporting that.
The incident highlights how, under the current patchwork of federal regulations, e-cigarette makers are mostly left to police their own manufacturing processes. The FDA does inspect some facilities to see if they comply with certain rules, including advertising and labeling laws. But it has not set tobacco industry–wide standards for manufacturing, despite being required to do so. And none of the vape devices on the market, including Juuls, have FDA authorization to be sold. A May 2020 application deadline will set the official authorization processes into motion.
In his lawsuit, Breja alleged that, in early March, he learned of “contamination” in about 250,000 mint refill kits, the equivalent of 1 million pods. They were made by Alternative Ingredients, an e-flavor supplier in Greensboro, North Carolina, and shipped by Juul to retailers. He said the issue was first “brought to [his] attention” on March 7 and came up again in an executive team meeting five days later.
Breja claimed that he was asked to recover about $7 million from the supplier, and, through his lawyer, told BuzzFeed News in October that the rest of the unshipped pods were destroyed. Breja interpreted these events to mean that his then-employer viewed the manufacturing issue as serious.
Juul did not comment on why he was directed to recoup the amount. Alternative Ingredients did not return requests for comment.
According to Breja’s complaint, in a March 12 one-on-one meeting, his supervisor dismissed his suggestions to issue a recall or tell the public, saying that doing so would damage Juul’s reputation and sales. Breja alleges that, a week after voicing this and other concerns about product quality, he was fired in retaliation. The company claimed he was terminated partly because of how he had portrayed his previous credentials, but Breja argued the allegations against him were “intentionally invented” to “set an example for other employees that whistleblowing would cost them their careers.”
Breja’s accusations came at a time when the vaping industry is under fire for endangering the public’s health. Although Juul’s stated mission is to help adult smokers quit, and studies support the idea that e-cigarettes are effective for doing so, the company has been accused of marketing practices targeting children and teens and getting them hooked on nicotine, in at least one case fatally. The $24 billion San Francisco startup is partially owned by the tobacco giant Altria and, as of September, run by a former Altria executive.
In addition, more than 50 deaths nationwide have been attributed to vaping-linked lung illnesses. The vast majority of these cases are linked to marijuana-containing devices, not nicotine-only ones like Juuls, although the CDC still warns against using vape devices of any kind.
On Nov. 7, a subcommittee of the House Committee on Oversight and Reform asked Juul and Alternative Ingredients to turn over documents and information about the alleged contamination. Chair Rep. Raja Krishnamoorthi expressed concern at the time that, given the widespread underage use of e-cigarettes, “any contaminated pods would disproportionately put children at risk.”
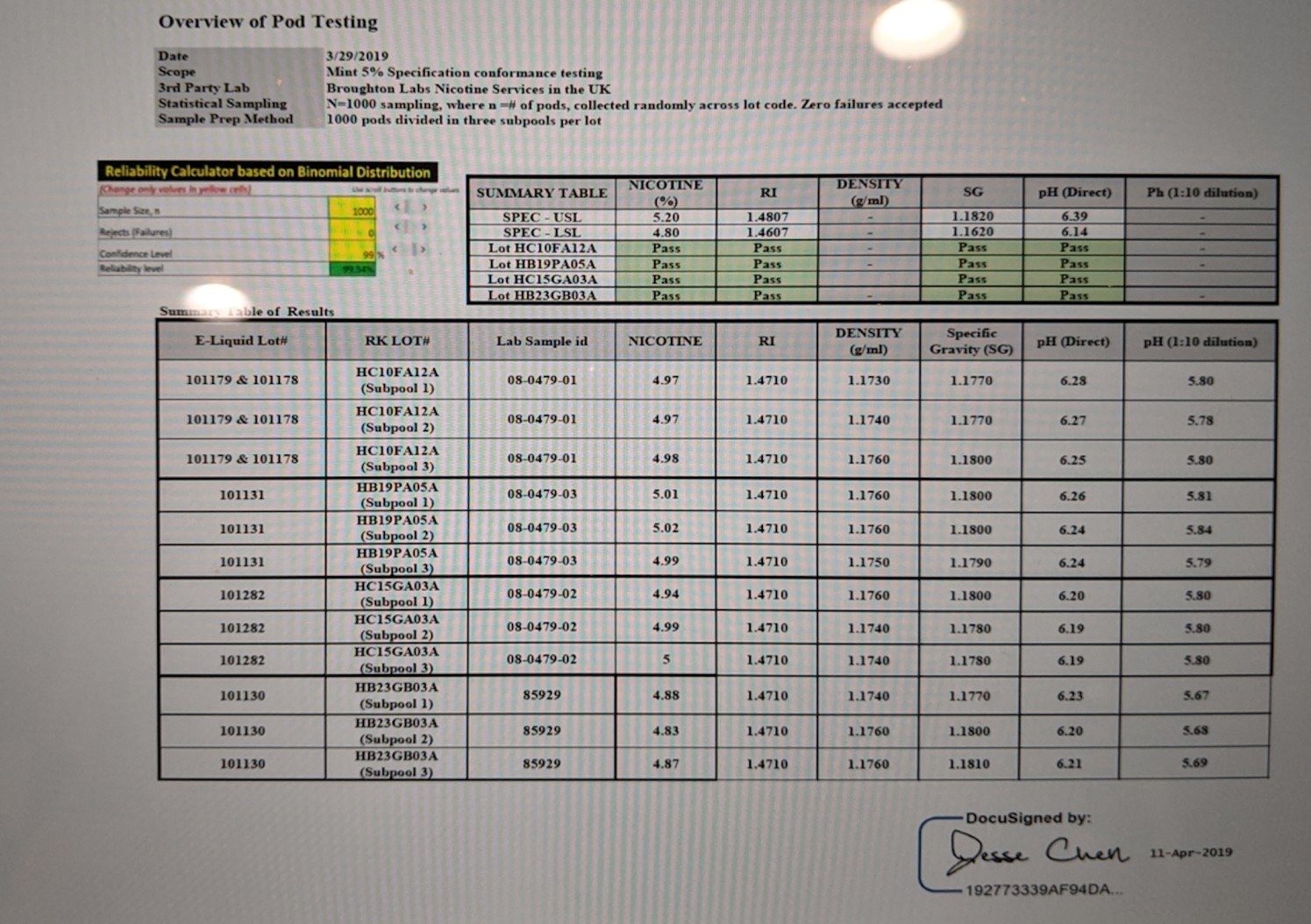
Juul’s internal report describes a visual “separation” incident involving a batch of about 240,000 mint refill kits, the equivalent of nearly 1 million pods, that were made by Alternative Ingredients. According to the report, the batch of 5% nicotine pods was shipped throughout the country on Feb. 26, and a product quality team at the company was told of the issue on Feb. 28. In his lawsuit, Breja said he learned about the “contamination” about a week later.
Finan, Juul’s spokesperson, said that a thin layer of flavoring did not fully mix into the e-liquid. The result was what the internal report described as a “visible three-layer separation.” The company halted shipment and began an evaluation of what was already in the marketplace, Finan said.
A sampling of inventory was sent for testing to Broughton Laboratories, a British company described on its website as offering “analytical, scientific and regulatory services” for e-nicotine companies. Juul did not answer questions about why the samples were sent outside the United States, and Broughton Labs did not return requests for comment.
Results from those tests, conducted in March, show that a sampling of the pods was tested for a half dozen characteristics. These tests were cited by the report as the basis for Juul’s determination that “injury is not reasonably foreseeable” and “no adverse health consequences will occur.” A health risk analysis scored the incident’s risk hazard index as a 1, or “negligible,” on a scale of 0 to 12.
The internal report says that separation also affected several other mint e-liquid batches, these containing 3% nicotine, that came to Juul’s attention after Breja left the company. This set of issues was not mentioned in the lawsuit, and lab tests did not find grounds for health concerns in either case.
If Juul users noticed something off about their mint pods around this time, very few apparently made their displeasure known. From the end of February to the end of July, according to the report, Juul received 143 complaints per 3.2 million pods distributed — a rate of essentially zero. There were also no serious injuries reported.
In his lawsuit, Breja made other claims about his time at the company that are not addressed in the report and that BuzzFeed News has not been able to confirm or deny.
Breja allegedly suggested to higher-ups that one-year-old pods should be labeled with an expiration date if Juul was going to resell them. In response, he claims that then-CEO Kevin Burns said, “Half our customers are drunk and vaping like mo-fo’s, who the fuck is going to notice the quality of our pods.”
Through a representative, Burns has denied saying this or “anything remotely close to this, period.” He did not respond to a request for comment for this story.