
Lynn just wanted to get back to normal. She wanted to feel like the woman she was before breast cancer.
So, in 2017, five years after her double mastectomy, Lynn got breast implants.
“I thought this would help me regain my femininity,” the 53-year-old told BuzzFeed News.
Instead, she unknowingly put herself at risk of getting cancer again.
Millions of breast implants, including Lynn’s, were recalled in July after they were linked to a higher risk of developing breast implant–associated anaplastic large cell lymphoma [BIA-ALCL], an uncommon cancer that has sickened hundreds of people and killed at least 33 others.
The Irish pharmaceutical giant Allergan agreed to recall more than 30 styles of its Biocell brand textured breast implants and tissue expanders after federal health officials linked the devices to a vast majority of cases of the disease.
But now, despite the recall, women are having difficulty receiving a proper diagnosis, leaving them scared, anxious, and potentially at risk of developing advanced stage cancer — an issue experts say is due to a lack of knowledge of the rare disease within the medical community. As a result, women have become their own advocates, sharing stories in private Facebook groups and helping others get answers.
“It’s been hell,” said Lynn, who was told by her doctors that she is their first patient to inquire about the breast implant–associated disease. “I had to notify them of the recall, I had to notify them what type of tests I need done ... I had to tell them about what BIA-ALCL is.”
Breast implant–associated ALCL is a cancer of the immune system that develops in the scar tissue that forms around the implant after surgery. It’s not yet known exactly why the implants may cause the disease, but physicians believe it involves the body’s response to their textured surface.
The disease is usually treatable when caught early by removing the implant and the scar tissue, also known as the capsule. In advanced cases, chemotherapy and radiation therapy may be required to get rid of the lymphoma. If left untreated, the disease can spread to other parts of the body and lead to death.
The first case of breast implant–associated ALCL was reported in 1997, but it wasn’t until January 2011 that the FDA acknowledged a possible association between breast implants and the disease. At that time, the US regulatory body had identified 34 cases worldwide.
Since then, physicians have diagnosed hundreds of women with the disease, further linking implants to the lymphoma. As of October, physicians based in the US had reported nearly 300 cases of the disease to PROFILE, a national registry of BIA-ALCL cases.
Yet, eight women who either have been diagnosed with the disease or are seeking a diagnosis told BuzzFeed News their doctors never mentioned the potential cancer risk when they underwent the implant procedure. Four of them were implanted with Allergan’s textured devices after the FDA reported the possible link in 2011.

“I had my breasts taken out for cancer. I didn't want to put cancer back in,” said Lynn, who wished to go by her middle name due to privacy concerns. “If they would have had warnings back then I would never have had implants put in. I would have been just happy with nothing.”
For many women, the cancer first presents several years after surgery with a large accumulation of fluid called a seroma, which can cause breast pain and swelling. Some will also develop a mass adjacent to the implant, swollen lymph nodes, skin rashes, and fatigue.
If doctors suspect that a woman has developed the lymphoma, they should order an ultrasound or MRI scan to look for a seroma or mass, according to national cancer guidelines. If either or both is detected, it can be aspirated or biopsied and tested for markers of the disease.
But some doctors aren’t following those protocols, and others are making mistakes by not conducting all the necessary tests or not collecting enough samples to have confidence in the results. In other cases, women said physicians who have little knowledge of breast implant–associated lymphoma aren’t listening to their concerns about the symptoms they’re experiencing.
“It’s like the hardest thing ever to just get someone to even take you seriously,” said Elizabeth, a 48-year-old woman from Wilmington, North Carolina.
Elizabeth, who asked to be identified only by her first name due to privacy concerns, started experiencing pain and swelling in her left breast in 2011, eight years after she got the textured implants.
“It just progressively got worse, but I was told it was OK,” she said.
In May, she left her job because she couldn’t work through the pain anymore.
By the time she learned that her implants had been associated with cancer in July, Elizabeth had developed a slew of other symptoms, from drenching night sweats to a swollen lymph node in her neck.
She recently dug into her medical records and discovered that in 2011 doctors had found a pocket of fluid in one of her breasts, but didn’t mention it to her or take any medical action. After learning about the disease, she took her concerns to her plastic surgeon, but he dismissed them.
“He used the word, women get all ‘emotional’ about this, and [said] ‘it’s just so rare,’” Elizabeth recalled. “And he said, ‘I’ve never seen it in my lifetime and I never will.’”
Paula Markham, a breast cancer survivor, heard about breast implant–associated ALCL in March when a friend alerted her to a segment about the disease on NBC’s Today show. At the time, the 56-year-old Knoxville, Tennessee, resident had a rash on her left breast and was dealing with extreme fatigue and pain on both sides of her chest, so she called her plastic surgeon's office to ask about the possibility she might have the disease.
“They didn’t think it was anything to worry about,” she told BuzzFeed News.

A couple months later, Markham found a quarter-sized skin lesion on her left breast, and made an appointment to see the surgeon. He diagnosed her with psoriasis, a chronic skin condition, and told her that the breast pain was normal.
Instead of ordering an ultrasound or an MRI, Markham’s surgeon gave her a steroid injection for the pain and told her to return for additional injections if it didn’t subside.
“I told him at that time, I said, ‘I really just want to get these out. I’m hearing bad things about them, I don’t feel good about them, I’ve had problems pretty much since I’ve had them put in. I just really would like to get them out.’ And he just kind of brushed me off and said, ‘You won’t be happy with the way you look,’” Markham said.
Dr. Mark Clemens, an associate professor of plastic surgery at the University of Texas MD Anderson Cancer Center, told BuzzFeed News he has seen patients with breast implant–associated ALCL who were misdiagnosed with other cancers, an issue he blamed on a lack of education.
Doctors “may still be unaware of this disease and may not know to test for it, so that can be a problem,” said Clemens, who has published more than 40 journal articles on the disease.
One woman from the San Francisco Bay Area, who did not want to be named, told BuzzFeed News she was misdiagnosed with and treated for inflammatory breast cancer, an aggressive cancer that blocks the lymph vessels in the skin, causing the breast to become swollen and red.
It wasn’t until after 18 rounds of chemotherapy and a unilateral mastectomy that her doctors realized she never had breast cancer. Instead, she had breast implant–associated ALCL. By the time she received the second diagnosis, the lymphoma had spread to other parts of her body.
“It underscores the importance of proper diagnosis and testing,” Clemens said.
To date, there are no known cases of women with smooth breast implants developing the rare cancer. And while textured implants are more popular in other countries, they represented less than 10% of breast implants sold in the US last year, according to the FDA. Macro-textured implants, the type sold by Allergan that have since been recalled, represent less than 5% of breast implants sold in the US.
It’s unknown how many women may be impacted by the recall. More than 4 million recalled devices are in distribution worldwide, but it wasn’t clear whether that figure included implants and tissue expanders already implanted into patients. Allergan declined to answer BuzzFeed News’ questions about the number of those impacted.
Unlike breast cancer, there is no screening test for asymptomatic women to determine whether they have a higher risk of getting the disease.
The disease has sickened women with both saline and silicone gel-filled implants, including those who got implants for reconstructive purposes after breast cancer or to prevent it.
“The common factor in these women has really only been that they've all had a textured implant at some point in their history,” said Dr. Colleen McCarthy, a reconstructive plastic surgeon at Memorial Sloan Kettering Cancer Center in New York and a principal investigator for the PROFILE database.

According to the FDA, the risk of developing breast implant–associated ALCL with Allergan’s macro-textured implants is about six times higher than with textured devices from any other manufacturer in the US.
“Continued distribution of Allergan's BIOCELL textured breast implants would likely cause serious, adverse health consequences and potentially death from BIA-ALCL,” the FDA said in a July statement about the recall.
The agency recently recommended that all manufacturers of breast implants — textured and smooth — incorporate a boxed warning and provide patient checklists to better inform women about the potential risks from implants, including breast implant–associated ALCL, implant rupture, and fatigue.
Yet despite the warnings, neither the FDA nor national plastic surgery associations recommend that women who are not currently experiencing symptoms have surgery to remove the recalled implants and their capsules.
Nor does Allergan, which will cover up to $7,500 in out-of-pocket surgery costs only if a woman has been diagnosed, and up to $1,000 in out-of-pocket costs for diagnostic testing in the case of a late-onset seroma. The manufacturer is also offering to cover the cost of smooth breast implants for those who want to remove their recalled devices, but none of the women who spoke to BuzzFeed News wanted to put any type of implant back into their body.
Products liability lawyer Gregory Bentley, who has filed two lawsuits against Allergan on behalf of women and has been retained by another 300 in the wake of the recall, called the company’s decision not to pay for the costs of removal for all women “unacceptable.”
“They're basically saying live with it, and if you do get diagnosed, we’ll give you $7,500 — that doesn't even come close,” Bentley told BuzzFeed News, adding that the surgery to remove the implants and capsules along with hospital fees can cost upwards of $20,000.
Clemens said there is no evidence that removing the implants and any scar tissue that has developed around it in women who have not been diagnosed reduces their risk of getting the lymphoma in the future.
“We have seen patients develop the disease after explantation, after implant exchange, and after total capsulectomy,” he said. “It is uncertain if there is any type of risk-reducing procedure and therefore it is discouraged by the FDA and our national societies.”
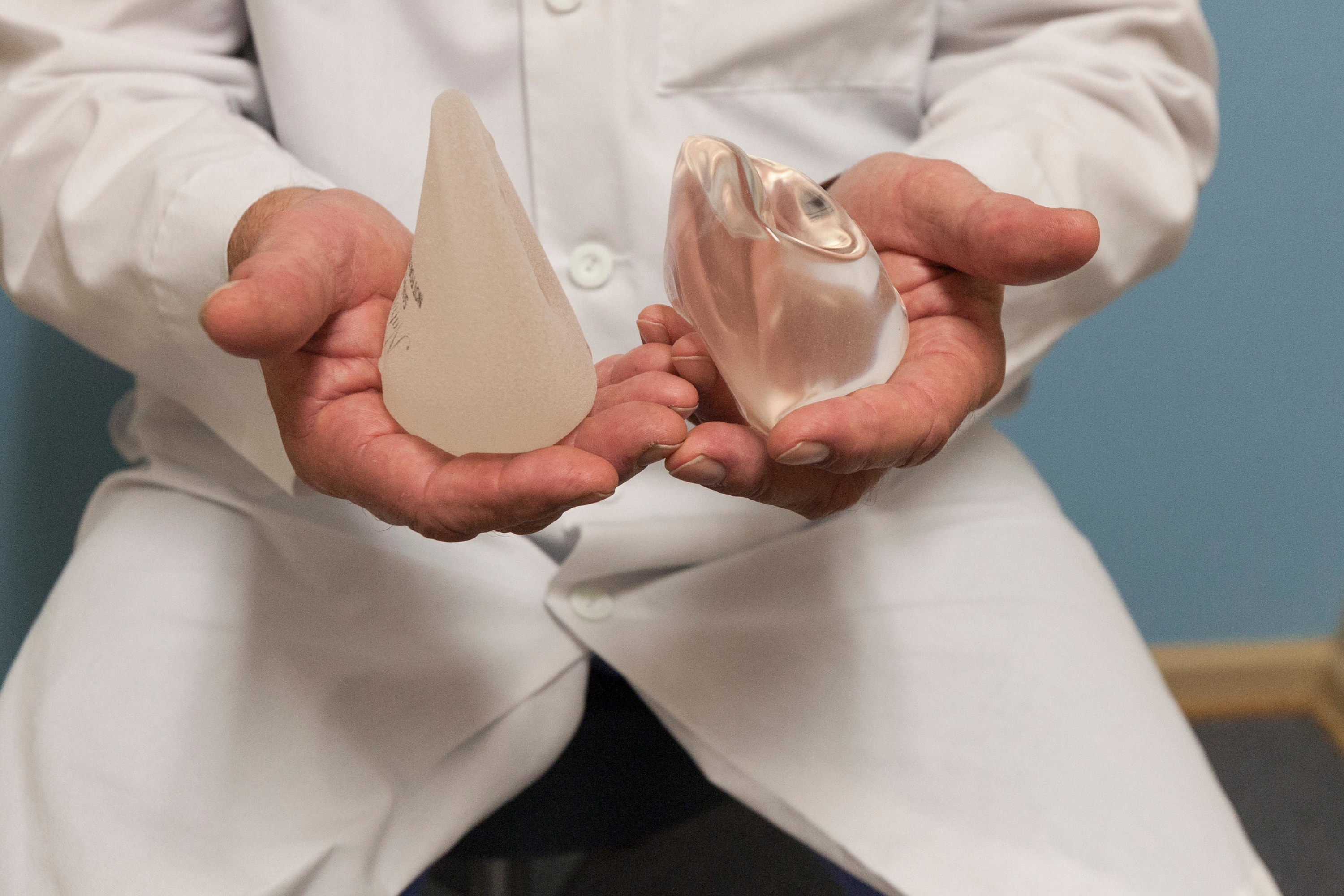
But some women who don’t have a large enough seroma or a mass to test are moving ahead with surgery anyway and asking their surgeons to examine everything they remove.
That’s what Michelle Forney did in January 2018 after pathologists failed to properly test fluid that had accumulated in her right breast nearly 20 years after she got implants.
“When he got in there, my right capsule had a bunch of tumors in it,” said Forney, 46, of Sacramento, referring to her plastic surgeon. “He sent it away and then it came back positive a week later.”
After getting pushback from her plastic surgeon in Knoxville, Markham turned to a private Facebook support group where Forney and other patients turned advocates swap advice and offer recommendations for surgeons who can perform the removal surgery.
“Why do I want to sit with something in my body that has been recalled,” she said. “It’s like telling you you’ve got faulty brakes and they’re going to recall them on the cars that are sitting on the car lot, but if you’re driving it don't worry about it.”
Now, she’s waiting to have her implants and capsules removed next month by a surgeon in Little Rock, Arkansas, an eight-hour drive away from where she lives.
“I’ve already had cancer and now I’m scared to death that I could possibly have cancer again,” Markham said. “Until I get them out and they are sent away for pathology and I get a definite no, I don’t think I’m going to be totally at ease.”
Connecting with other women who are going through the same ordeal in the Facebook group has helped. They’ve shared symptoms, offered tips for procedures and tests to ask for, and provided support in their fight for answers.
“If it wasn't for the support group on Facebook, I would not have known anything,” said Lynn, who convinced her primary care doctor to order scans that detected a seroma and two masses after her plastic surgeon refused.
The masses were both benign, but not enough fluid was collected to test for the lymphoma. Lynn said she plans to get more testing when she has her implants removed next month. If she's diagnosed and has to go through another round of chemo, Lynn said she and her husband would likely need to sell their family business.
“I’m just trying to put things in order and I'm trying to be like, what’s the best-case scenario, what's the worst-case scenario?” she said. “We’ll probably have put it up for sale and then just love each other and hope for the best.”
