
Every September, Lindsay Chapman, her husband Andrew Nesseth, and the state of Minnesota celebrate the little boy they lost in 2010.
Leo was born with Alper’s disease, caused by a rare genetic mutation that damaged his mitochondria, the oblong structures that generate a cell’s energy. At 9 months old he had a seizure, the beginning of an episode that eventually paralyzed his right side. Then his liver began to fail. On his first birthday, his parents took him to the Minnesota Zoo, where he gleefully rode the monorail, tasted chocolate frosting for the first time, and got drenched in a surprise rainstorm. Three days later, he died.
Chapman and Nesseth did everything they could to bring the public’s attention to mitochondrial diseases, which affect up to 4,000 babies in the US each year. The couple drummed up social media campaigns, spoke at hearings, baked cookies with mitochondria traced in green icing, and passed out little green light bulbs at the public library of their tiny Minnesota town. Their efforts culminated in a 2011 state law, known as “Leo’s Law,” which designates the third week of this month for mitochondrial disease awareness.
Unlike most genetic syndromes, which can be passed down from mother or father, mitochondrial diseases usually stem from a mother’s egg. They’re very rare: Every year in the US, about 100 women find out they carry these genetic glitches, which cause a wide array of symptoms, from hearing loss and migraines to muscle and liver failure, or even death.
Mitochondrial diseases have no cure. But a controversial genetic technology can prevent a woman from passing these mutations on to her kids. It’s a type of in vitro fertilization in which the faulty mitochondrial DNA inside a woman’s egg gets swapped out with mitochondrial DNA from a healthy donor, leading to a child who technically has three genetic “parents.”
The technology was approved in the UK last February, but in December, US lawmakers took the opposite stance, slipping language into the Consolidated Appropriations Act of 2016 — the big annual spending bill — that banned the FDA from evaluating clinical trials for any genetic modifications that affected the next generation.
Now that law is due to expire, but scientists and patient advocates are worried that the FDA ban will be quietly renewed without discussion or debate.
“There is something unusual, perhaps disturbing, about Congress laying down the law when the scientific community and public are just beginning to understand the issue,” Eli Adashi, a professor of medical science at Brown University who has been documenting the regulation of politically sensitive science for the last two decades, told BuzzFeed News.
Congress’s actions are at odds with the momentum of the FDA, which for the last two years has been gearing up to approve tests of mitochondrial replacement in people. Counting on this approval, a handful of scientists in the US had begun to set up these clinical trials. But now that work is indefinitely on hold.
“I can’t even fathom why they would think that that would something we shouldn’t be researching and frankly, doing clinical trials on,” Chapman told BuzzFeed News. “There’s just something inside of me that screams at the idea that somebody else would stand in the way.”
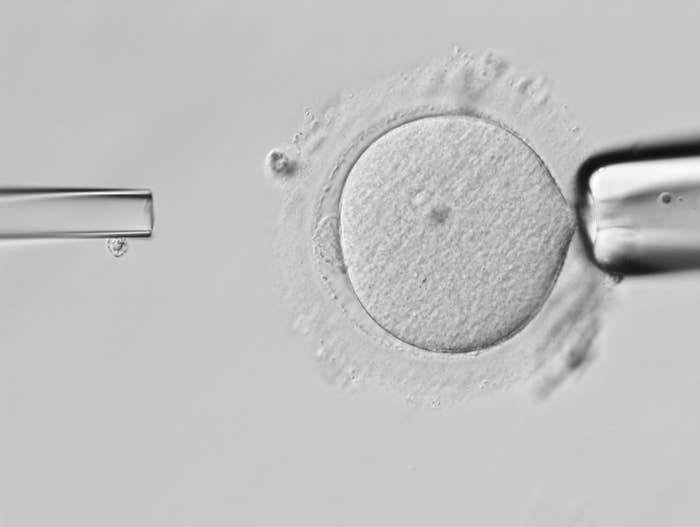
Before the ban was passed, at least two clinical trials had been planned to test mitochondrial replacement therapy in eggs harvested from women with the disease.
One method, which involves replacing the mitochondria of a woman’s egg cell before it is fertilized with sperm, had been tested in animals, but not people.
“To implant the embryos and follow the children after birth — that is the only part that’s left unknown,” Shoukhrat Mitalipov, director of the Oregon Health and Science University Center for Embryonic Cell and Gene Therapy and leader of the research, told BuzzFeed News.
Mitalipov said that in early conversations with the FDA he had proposed a trial with up to 10 women who had mutations in their mitochondrial DNA that coded for MELAS, a disease that affects the nervous and muscular systems. But the ban foiled his plans.
“It’s definitely going to slow down clinical translation and that’s unfortunate,” Mitalipov said. “Even though the US was one of the pioneers in developing these treatments, it looks like clinical implementation will be somewhere in the UK and probably in other countries.”
On the other side of the country, Michio Hirano, a professor of neurology at the Columbia University Medical Center, had been working on a different technique, in which a mom's non-mitochondrial DNA is transferred into the egg of another woman with healthy mitochondrial DNA.
“We were certainly hoping to use the technique clinically,” Hirano told BuzzFeed News. “It’s a bit disappointing, but it doesn’t appear that we will be able to do it clinically in the near future.”
Hirano is still planning to test mitochondrial replacement in women’s eggs in the lab. But to work around the letter of the law, he will freeze the resulting fertilized embryos instead of putting them back in the women with mitochondrial disease. If and when the ban lifts, they will be ready for implantation.
“We would like to preserve the embryos indefinitely until the FDA approves,” he said. “It’s the only way that we can proceed.”
Patients with mitochondrial disease are frustrated about the ban’s chilling effect on research.
Patients with mitochondrial disease are frustrated about the ban’s chilling effect on research. “That effectively squashed any activity in this space,” Philip Yeske, science officer at the advocacy group the United Mitochondrial Disease Association, told BuzzFeed News.
The ban is particularly baffling, he said, because the National Academies of Sciences had thoroughly investigated the technology’s ethical and safety implications and ultimately offered its support of clinical trials.
“They were very clear in their report — they saw no ethical reason to limit human clinical studies for mitochondrial replacement therapy,” Yeske said.
Still, the new method is not without its scientific detractors, who have warned that mixing mitochondrial and non-mitochondrial DNA from two females can shorten the lifespan of some animals including fruit flies and mice. And even the National Academies report suggested some precautions. Among them, it recommended that researchers start with doing the tests in male embryos. That way, if there were any mishaps in the way the transfer took place, the boys would be in no danger of passing it down to their own children.
Adashi says that it’s “a good possibility” that the bill’s primary target was not mitochondrial replacement, but a more contentious genetic technology called CRISPR. Unlike mitochondrial replacement, which simply swaps out whole strands of existing DNA with DNA from another person, CRISPR targets specific genes in the code to delete or “edit.”
Scientists have been fiercely debating the ethics of using CRISPR on human embryos and critics worry that this could lead to the creation of “designer babies.”
The language in the December bill prevents the FDA from considering, or even acknowledging, trials of any technology that would alter the genome of the next generation.
So this language also blocks research into mitochondrial replacement therapy — a technology that is more mature, better tested, and less ethically fraught than gene-editing like CRISPR.
“The mitochondrial replacement can be considered a form of genetic modification but it’s not gene editing — it’s not that we go in and fix a mutation or edit the DNA,” Hirano said.
Adashi, the Brown professor, told BuzzFeed News that he did not know how the ban’s language, which is simple but specific, came to be a part of the bill. “There’s no paper trail, there’s no smoking gun — there is just the result,” he said.
In an email to BuzzFeed News, the Appropriations Committee spokesperson Jennifer Hing would not comment on where the language came from.
The law expires on September 30, but a “continuing resolution” is expected to pass this month that would keep these provisions relevant until the first week of December. Scientists like Adashi and Yeske worry that the same language will be included in next year’s spending bill without any discussion.
After Leo died, Lindsay Chapman and her husband struggled with the prospect of having another child. Leo’s mitochondrial disease was one of the rare types that comes from a mutation in regular DNA, which can be inherited from either parent. So Chapman and Nesseth knew that there was a 25% chance their next child would have it.
“It’s been six years of conversations —very difficult conversations,” Chapman said. Her husband definitely wanted another child, she said. “And I couldn’t even fathom the idea.”
And then last year, Chapman found out she was pregnant again. She was devastated.
“At first, I felt like I couldn’t keep the pregnancy. I couldn’t deal with the stress,” she said. Nesseth convinced her to wait out the 14 weeks until they could test the fetus for signs of the defective gene. Fortunately, the tests were negative, and baby Nikolai was born this spring.
Although they would not benefit from mitochondrial replacement therapy themselves, they are in full favor of any procedures that could give mothers a reliable chance to have healthy children.
“I don’t think that people who are making up these laws and deciding where the money goes really understand the journey that families go through, and the stress that they go through to potentially grow their families,” she said. “To even have the opportunity to have an unaffected child is amazing.”
