An independent CDC expert panel on Wednesday put off a vote on whether the pause of Johnson & Johnson’s COVID-19 vaccine should continue, citing a need for data expected to come in the next two weeks on the shot’s links to an extremely rare and dangerous blood-clotting illness.
The emergency session of the CDC’s Advisory Committee on Immunization Practices (ACIP) panel was held after reports of six patients who developed blood clots after getting vaccinated. This led federal health officials on Tuesday to recommend “out of an abundance of caution” that states pause their administrations of the shots.
Asked to assess the shot’s safety, risks, and appropriate use, the panel punted the decision on whether it should be resumed for one to two more weeks, citing a lack of enough data beyond six confirmed cases reported by federal health officials on Tuesday.
“We are not ready to vote at this time,” said panel chair José Romero of the Arkansas Department of Health. Another meeting of the group will be scheduled by Friday.
Their indecision reflects the thorny public health dilemma before regulators in the US as well as the rest of the world.
A similar clotting syndrome has been reported among more than 100 recipients of the AstraZeneca vaccine in Europe, which uses a technology similar to J&J’s. The condition is thought to perhaps result from the vaccine triggering an allergic reaction against a protein in blood platelets under very rare circumstances, leading to blood clots that might cause strokes or other problems.
Already, reports of clots have led some countries, including Italy, Spain, and Belgium, to restrict the use of the vaccine to older people, while the UK has said that healthy, young people who are not at high risk of COVID-19 should have the option of a different vaccine if one is available.
In the US, the reports of alarming side effects from the J&J vaccine — which has been given to about 7 million people since late February — lead to tough questions: whether it is in the public’s overall best interest to continue to administer the shot, restrict it to some people, or cancel it altogether.
On Wednesday, many ACIP panel members said they thought the best way to instill public trust would be to collect more data about the cases and underlying science before making a call. “By having a little more robust information, I think we can be much more confident in how we talk about the safety of this vaccine,” said Lynn Bahta of the Minnesota Department of Health. “Right now the confidence for COVID vaccines is just right at a precipice. We’ve got people that can’t wait to get it, and others who have been waiting and seeing. This contributes to that confidence that people have.”
But other members, even those who acknowledged that more data would be ideal, pointed out that hundreds of people are dying daily from COVID in the US, and that the single-shot J&J vaccine, which is easy to administer to people in hard-to-reach locations, was supposed to be a key tool in ending the pandemic. Some worried that extending the pause would be a death knell for the immunization.
“Putting this vaccine on pause for those of us who are frontline workers has been devastating,” said Camille Kotton, an infectious disease physician at Massachusetts General Hospital. “I agree in general that we don’t have enough data to make a decision at this time, but we were planning on using this vaccine in the state of Massachusetts for people who are homebound and otherwise not able to get a vaccine.” Suspending use of the vaccine for any period of time is a “significant loss” for those underserved populations, she said.
FDA official Doran Fink suggested that the vaccine could continue to be distributed as long as doctors and recipients were better informed about the signs and risks of the clotting syndrome, so anyone experiencing the side effect could get the correct treatment right away. The option was discussed by the panel, but the experts felt uncomfortable embracing it for now.
At a White House briefing earlier on Wednesday, federal officials said that enough doses of the Moderna and Pfizer vaccines are available to vaccinate the US population, even without the J&J shots. More than 192 million of those doses have been administered in the US in total and are continuing at a rate of 3.3 million a day. There are roughly 13 million doses of the J&J vaccine waiting for administration either at clinics or in delivery, according to the CDC.
The side effects were reported after more than 6.8 million doses of the J&J vaccine had been given in the US. Of them, at least six women under the age of 50 have been reported to develop cerebral venous sinus thrombosis (CVST), or brain clots, along with low levels of blood platelets — an unusual combination, since platelets are key to forming clots that stop bleeding. One of the women died, and three are still hospitalized.
More cases may come to light over the next one to two weeks, CDC officials suggested at Wednesday’s meeting, given the two-week window in which cases have occurred so far. Symptoms of the cases included headaches, leg pain, backaches, and leg swelling.
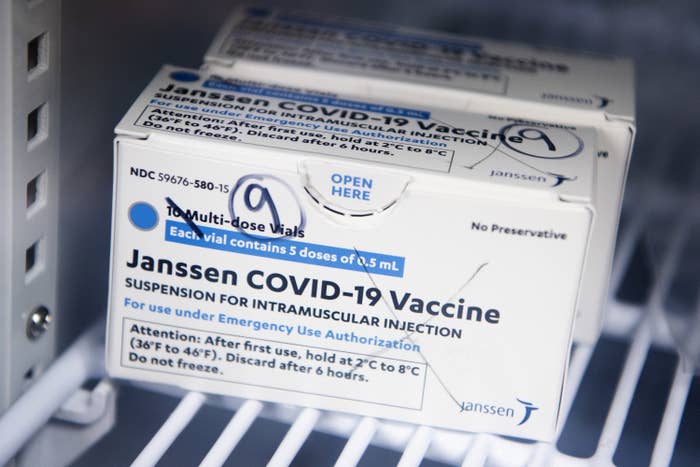
Representatives from Janssen, the pharmaceutical arm of J&J, relayed on Wednesday that the federal vaccine safety system had reported a likely seventh case in a 28-year-old woman, with details still being collected. Information on her platelet levels was not available at the time.
In the six confirmed cases, which were detected by the US’s vaccine adverse event reporting system, the symptoms occurred 6 to 13 days after getting the shot, according to the CDC. The agency has called for doctors to watch out for patients developing blood clots or low blood platelet counts after getting the J&J vaccine. Typical treatment for this type of clot, a blood thinner called heparin, may harm patients and should be avoided, the agency warned.
Heparin was given to four of the six women in question, three of whom had not recovered as of mid-April, Janssen said on Wednesday. It was not clear whether heparin caused the one death, CDC officials said.
During J&J’s clinical trials, one vaccine recipient — a 25-year-old man — developed, then recovered from, similar symptoms to the clots now being reported.
Both the J&J and AstraZeneca vaccines rely on a harmless virus to deliver the coronavirus spike protein to immunize people, as do Russia's Sputnik vaccine and China’s CanSino Biologics vaccine. This "adenovirus" class of vaccines is particularly useful in immunization efforts because it only requires standard refrigeration and, in J&J’s case, just one shot. There is now concern that this class may uniformly carry a risk of clotting side effects, however “extremely rare” they may be, as federal officials emphasized this week. No cases have been seen in earlier Ebola or respiratory syncytial virus vaccines that use the technology, according to Janssen.
The technology is different from the one underpinning the Pfizer and Moderna vaccines, both of which use mRNA to teach the body how to make a protein that triggers an immune response to the coronavirus. No cases of CVST with low blood platelets have been reported among people who have received Moderna or Pfizer vaccines.
At Wednesday’s meeting, Janssen representatives argued in favor of continuing to administer the vaccine to the population at large while telling doctors about the symptoms and treatment of the clotting syndrome. “Based on the current data, Janssen believes the overall benefit-risk profile for our vaccine is positive across the population for which it’s authorized,” Aran Maree, Janssen’s chief medical officer, told the ACIP.
But CDC official Sara Oliver put forth the case for extending the pause for a limited period of time or restricting the vaccine on the basis of gender or age, such as to only men or only people above age 50. She pointed out that the number of these blood clots reported so far, while still very rare, exceeds the number that would be expected to normally occur among young women. Oliver also noted that the country’s supply of Pfizer and Moderna vaccines is expected to remain steady for the foreseeable future.
“The decision isn't necessarily receipt of a Janssen vaccine versus remaining at risk for COVID,” she said. “The decision may be receipt of a Janssen vaccine versus receipt of another mRNA vaccine.”
UPDATE
This post has been updated to include the daily number of deaths from COVID-19 in the US.

