Fourteen years after approving a surgery-free sterilization device called Essure, the FDA has announced that the device can come with dangerous side effects, and is demanding new safety trials.
The agency will accept public comments on the language of a new black box warning, the strongest warning label the agency can require.
But many women who claim to have suffered serious complications after having the device inserted — including pain, perforation of the uterus, and even pregnancy — say that the FDA’s new recommendations are too little, too late.
“We are outraged that it appears as if the FDA is going to leave Essure on the market,” said a statement released by Essure Problems, a coalition of over 27,000 women that started as a Facebook group to discuss the device’s side effects. “These studies could take several years, and leaving the device on the market will only put more women's lives at potential risk.”
The FDA’s guidelines will likely change in response to comments from the public and the device’s manufacturer over the next 60 days. But as they stand today, the guidelines would require the manufacturer, Bayer AG of Leverkusen, Germany, to run a new drug trial that compares the device’s risk of serious complications to the side effects of tubal ligation — the standard, surgical option for female sterilization.
“While the FDA believes Essure remains an appropriate option for the majority of women seeking a permanent form of birth control, some women may be at risk for serious complications,” the FDA said in the statement. “These may include persistent pain, perforation of the uterus or fallopian tubes from device migration, abnormal bleeding and allergy or hypersensitivity reactions.”
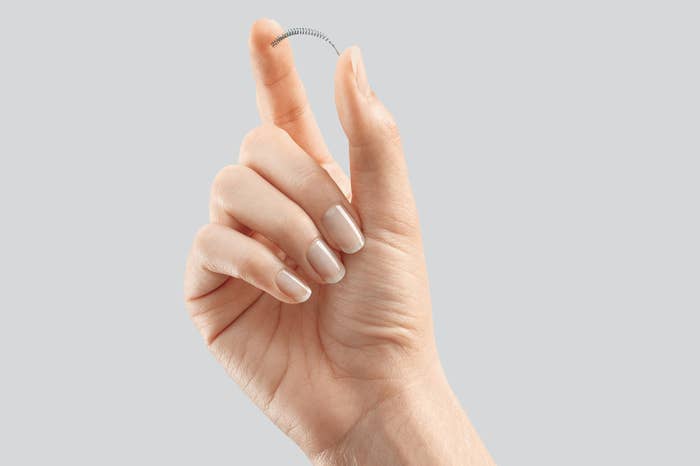
The device, which was approved in 2002, consists of flexible metal and polyester coils inserted into each of a woman’s fallopian tubes. The coils work by inducing scar tissue to form around them, effectively blocking sperm from getting to eggs. Unlike tubal ligation, Essure doesn’t require any anaesthesia or cuts, making it a popular non-surgical option. According to Bayer, the device has been inserted into 750,000 women to date.
“Essure is an important permanent birth control option with a positive benefit-risk profile and more than a decade of post marketing experience worldwide,” Bayer said in a statement sent on Monday to BuzzFeed News. “Bayer will continue to work closely with the FDA to support the safe and effective use of Essure.”
From 2002 to 2015, the FDA received more than 5,000 complaints about about negative side effects as a result of the device. The Essure Problems Facebook group is littered with painful anecdotes, pictures, and support about complications potentially due to the device.
Angie Firmalino, the group’s founder, told BuzzFeed News she had to have a hysterectomy and multiple painful procedures after the coils were expelled from her fallopian tubes. Women who get Essure are about 10 times more likely to need follow-up surgeries than those who get their tubes tied, according to one study.
Last September, in a highly unusual move for a drug that’s already been approved, the FDA convened doctors, patients, and representatives from Bayer to discuss problems with the device in front of an advisory committee for the agency. Several members of the Facebook group testified.
“A lot of people were hopeful and probably falsely optimistic and uncritical when the pre-marketing data came out,” Aileen Gariepy, an assistant professor of obstetrics, gynecology, and reproductive sciences at Yale School of Medicine who testified at the FDA meeting, told BuzzFeed News. “That is true for physicians like myself. That’s definitely how the data was presented by the company.”
Essure was vetted using the FDA’s strictest path to market, called pre-market approval. That means the device was subject to two clinical trials, unlike many other devices on the market. But the clinical trials didn’t include a comparison control, such as tubal ligation, to see how Essure stacked up against its competitors in terms of safety and effectiveness.
What’s more, women who had failed implants were removed from the study instead of being followed throughout the course of the trial, leading to a rosier picture in the data turned over to the FDA.
“We agree with the FDA that a new post-market study is needed,” Diana Zuckerman, president of the National Center for Health Research, told BuzzFeed News. “But we think the FDA should take over control of the study, because there’s so many questions about the previous studies on Essure being inaccurate and intentionally misleading.”
Because the device was developed as a permanent birth control option, women who have experienced serious side effects and wanted the devices removed have come across a big roadblock: there is no official protocol from Bayer on how doctors can remove the coils.
Some doctors will remove the coils through procedures like hysterectomies and cutting a woman’s fallopian tubes out. But Zuckerman notes that many recipients of Essure are poor women on Medicaid, and are less likely to have access to those doctors.
“When a low-income woman on Medicaid gets sterilized with Essure,” Zuckerman said, “if something goes wrong, the chances that she’ll find a physician who knows how to remove it safely are not very high.”

